Why does the same drug affect different people—or cells—in different ways? In medicine, it’s well known that patients can respond very differently to the same treatment. The same principle holds true in the microscopic world of yeast cells: depending on their genetic background, some are highly sensitive to a drug while others shrug it off. For a long time, scientists assumed that—even if sensitivity varied—the cause of death for a given drug was always the same.
Camptothecins: Breaking the Flow

But recent research challenges that assumption. In the case of camptothecins, a family of cancer drugs, the story is far more nuanced.
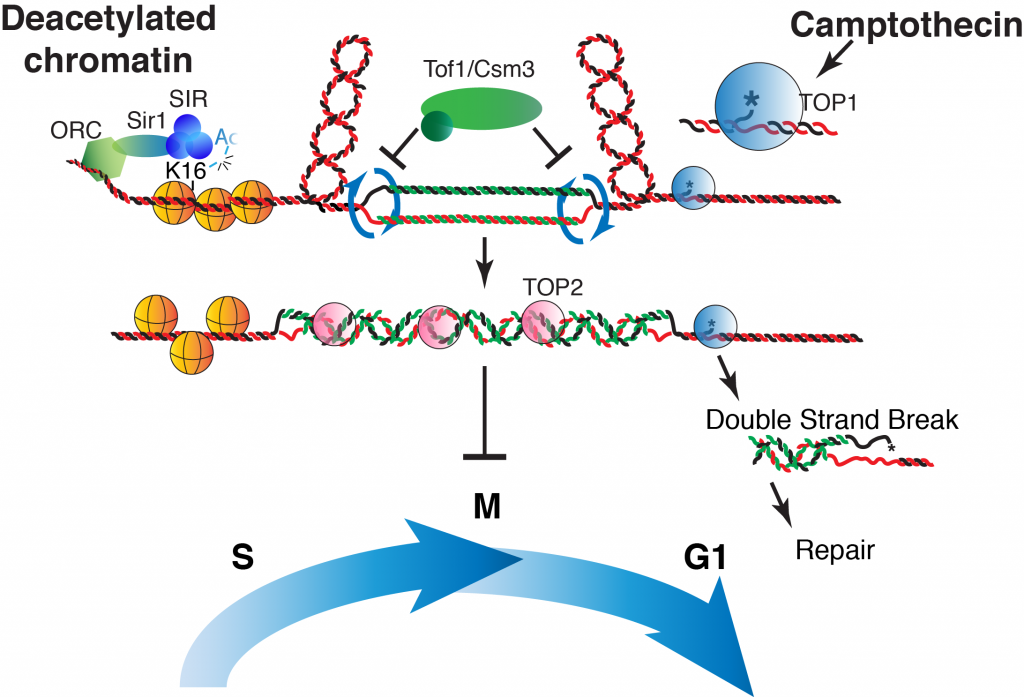
Camptothecins target Topoisomerase 1 (Top1), an enzyme that normally helps relieve the overwinding of DNA strands as they’re copied during replication. Top1 works by cutting one DNA strand, allowing it to unwind, and then resealing it—like carefully loosening a twisted rope.
When camptothecin steps in, it traps Top1 in a frozen embrace with DNA. Instead of a helpful “cut-and-release” cycle, Top1 becomes a roadblock—a physical barrier that stalls DNA replication.

Cells have one temporary workaround: they can rotate the replication fork, converting the overwinding into DNA catenanes—two daughter DNA molecules twisted around each other like chain links. This keeps replication moving, but it creates a new problem later: when the cell tries to divide, the tangled chromosomes must be untangled by Topoisomerase 2 (Top2).
For years, scientists thought the main reason cells die under camptothecin was because the blocked Top1 leaves behind DNA breaks. These double-strand breaks (DSBs) are indeed lethal when repair is impossible. But what about normal cells that can repair DSBs efficiently? Why are they still sensitive?
Silence in the Genome: The Hidden Culprit
My research revealed that sensitivity in repair-proficient cells isn’t primarily caused by too many breaks to fix. Instead, it stems from a special chromatin structure—one shaped by the SIR silencing complex.
This complex removes an acetyl group from a specific site on histone H4 (lysine 16), a process known as H4-K16 deacetylation. This “silencing mark” compacts chromatin into a more closed state. ORC and Sir1 help direct the SIR complex to specific genomic regions.

When camptothecin traps Top1, these compact silent regions act like extra roadblocks, amplifying the topological stress on the replication fork. Fork rotation—and thus DNA catenation—happens even more often, leaving daughter chromatids deeply intertwined. As a result, cells struggle through mitosis, wasting precious time in M phase as they try—and often fail—to fully untangle their DNA.
In essence, chromatin silence turns up the volume of camptothecin’s effects.
A Universal Whisper: From Yeast to Humans
The beauty of this discovery is its conservation. My team showed that the phenomenon holds true not only in budding yeast, but also in fission yeast and even human cells. Inhibiting silent chromatin formation—for example, with drugs that block sirtuin deacetylases—reduces sensitivity to camptothecin.
This means the relationship between silent chromatin and drug sensitivity could play a role in how cancer patients respond to camptothecin-based treatments.